PROCEDURE The test can be executed possibly in 5 original containers if sufficient volume of solution is accessible in Each individual container and the product or service container is usually entered aseptically (i.e., needle and syringe by way of an elastomeric rubber stopper), or in 5 sterile, capped bacteriological containers of suitable size into which a sufficient volume of merchandise is transferred. Inoculate each container with among the ready and standardized inoculum, and blend.
Among the list of important techniques in keeping away from the Peter Principle is fostering self-consciousness amid personnel. It is necessary for people to acquire a practical comprehension of their strengths, weaknesses and aspirations in an environment where it can be Harmless to do so. This isn’t usually straightforward, particularly when there is very little time for self-reflection.
The specified microorganisms have to be detected While using the indicator reactions as explained less than Testing of Products and solutions.
The non-selective agar is essential to determine the CFU concentration of your inoculum. The new batch of liquid media is suitable if:
Utilizing the calculated concentrations of cfu for every mL existing Initially from the test, calculate the transform in log10 values on the concentration of cfu for each mL for each microorganism on the applicable test intervals, and express the modifications concerning log reductions.
It is best to use the strains that happen to be cited On this chapter, or equal strains from other culture collections. By way of example, if Pseudomonas aeruginosa ATCC 9027 is indicated, you ought to use this pressure or strains from other society collections declaring equivalence to ATCC 9027. Other strains which include ATCC 14149 usually are not ideal.
I truly appreciated how I was dealt with by each of the staff at Anresco. As a small, very first time merchandise producer, regulations might be bewildering.
The Peter Principle, coined by Dr. Laurence J. Peter, states that folks in hierarchical organizations are inclined to rise for their amount of incompetence. To put it differently, individuals "are frequently promoted primarily based on their own effectiveness within their existing roles as opposed to their potential for achievement in greater positions.
– Staphylococcus aureus yang tumbuh harus berwarna putih/kekuningan dengan zona berwarna kuning di sekeliling koloni.
Take into account, the pharmacopoeias are certainly not harmonized for environmental checking and each has varying requirements that demand from customers pretty small Original contamination Restoration premiums or maybe the detection of very reduced cfu amounts (Tables one and a pair of). The requirements fluctuate depending upon the criticality of the manufacturing region to solution get more info sterility. With regards to the cleanroom classification, there can be quite stringent demands on the end result of environmental monitoring.
TSB is usually a non-selective medium, missing precise inhibitors or selective brokers to inhibit the growth of specified microorganisms although selling the growth of Some others.
GPT ought to be carried out within the more info media employed for recovering environmental isolates. It is strongly encouraged, and helps make for an even better evaluation in the microbiological high-quality in the media if environmental isolates are involved with GPT.
. Each time a new seed-inventory vial is required, it might be eliminated and utilized to inoculate a series of working cultures. These Performing cultures could then be used periodically (on a daily basis in the situation of microorganisms and yeast) to get started on the inoculum tradition.
For brand new whole lot broth media, Growth promotion, inhibitory and indicative test shall be completed only qualitatively to determine the efficacy of media.
 Alicia Silverstone Then & Now!
Alicia Silverstone Then & Now! Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Hailie Jade Scott Mathers Then & Now!
Hailie Jade Scott Mathers Then & Now!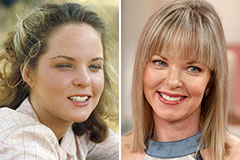 Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now! Morgan Fairchild Then & Now!
Morgan Fairchild Then & Now!